Background: The success of clinical trials of new therapies for sickle cell disease (SCD) will depend on the recruitment and retention of a large and diverse group of people with SCD (PwS). Qualitative studies have reported barriers to participation in clinical trials, including the potential impact on health, unmanageable study demands, limited knowledge of trials, and lack of trust in healthcare(Patterson CA et al. J Pediatr Hematol Oncol 2015; Lee LH et al. Blood Adv 2021). The Learning and Insights into Sickle Cell Trial Experiences (LISTEN) Survey was developed to provide a robust and comprehensive understanding of the global barriers and motivators to participation in clinical trials for PwS.
Aim: To present interim findings from the LISTEN Survey regarding participation and experiences of PwS in clinical trials, barriers and motivators to participation, and differences in responses from PwS compared with perspectives of healthcare professionals (HCPs) treating PwS.
Methods: Between October 6, 2022 and June 22, 2023, a broad range of adults (≥18 years) with SCD and HCPs involved in the treatment and/or clinical research of SCD in 17 countries were asked to complete corresponding quantitative surveys. PwS were asked whether they had participated in a clinical trial and, if so, whether their experience had met their expectations. PwS were asked to rate on a 7-point scale (from not at all to extremely important) and rank (from most to least important) the importance of specified factors (grouped into five categories) when deciding whether to participate in a clinical trial for SCD. HCPs provided their perspectives on the importance of these factors to PwS. The results presented here include the total proportion of respondents who rated factors as extremely or very important and the proportion who ranked factors first or second.
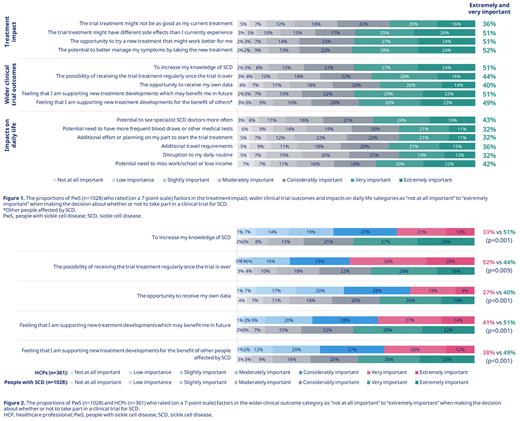
Results: Overall, 1028 PwS (57% female) with a median age (interquartile range) of 30 years (24-37) from 16 of the 17 countries and 361 HCPs (67% hematologists and/or SCD specialists) from 17 countries completed the survey. In total, 35% of PwS had been invited to participate in a clinical trial for SCD. In PwS who had taken part in a clinical trial (24%; n=249/1028), compared with their expectations, 36% reported a better experience, 36% the same as expected experience, 9% a worse experience, and 20% did not know what to expect. Extremely or very important factors that motivated PwS to participate in a clinical trial included the potential to better manage their symptoms (52%), the opportunity to try a new treatment that might work better (51%), and to increase their knowledge of SCD (51%; Figure 1). An important barrier to participation was the potential to experience different side effects (51%). In the trial information category, PwS ranked (first or second of five) safety measures (57%) and how the treatment works (49%) as the most important factors; these were considered to be significantly more important than who is leading (27%) or funding (24%) the trial; p<0.001 for all combinations. In the further considerations category, the majority of PwS ranked (first or second of five) speaking to other PwS involved in the trial (51%) and experts running the trial (50%) as the most important factors. HCPs overstated the potential practical barriers for PwS compared with responses from PwS, including missing school/work (50% vs 42%; p=0.009), the effort required to start the trial treatment (45% vs 32%; p<0.001), and travel requirements (52% vs 36%; p<0.001). HCPs understated the importance of wider clinical trial outcomes as motivators for PwS compared with responses from PwS, including supporting new treatment developments that may benefit them (41% vs 51%; p=0.001) or other PwS (38% vs 49%; p<0.001; Figure 2).
Conclusions: Improving access and recruitment into clinical trials in SCD will require clear communication of the potential benefits to individuals and the wider SCD community, as well as potential safety and side effects. Findings from the LISTEN Survey highlight that those directly involved in the trial should deliver these messages, including other PwS who are involved in the trial. Given the disconnect between PwS and HCPs, shared decision-making may also improve understanding and increase participation in clinical trials. Further analyses of the survey results will be important to identify the differences in responses between regions, age groups, and other subpopulations.
Disclosures
James:Sickle Cell Society: Current Employment, Other: CEO. Andemariam:American Society of Hematology: Research Funding; Emmaus: Consultancy; Bluebird: Consultancy; Connecticut Department of Public Health: Research Funding; GSK: Consultancy; Accordant: Consultancy; Novartis: Consultancy, Research Funding; HRSA: Research Funding; PCORI: Research Funding; Hemanext: Consultancy, Research Funding; Sanofi Genzyme: Consultancy; Vertex: Consultancy; Afimmune: Consultancy; Agios: Consultancy; NovoNordisk: Consultancy; Global Blood Therapeutics: Consultancy, Research Funding; Forma Therapeutics: Consultancy, Research Funding; Pfizer: Research Funding. Mahlangu:Catalyst: Research Funding; Sandoz: Research Funding; Novo Nordisk: Research Funding; Roche: Research Funding; Pfizer: Research Funding; Spark Therapeutics: Research Funding; Sanofi: Research Funding; BioMarin Pharmaceutical Inc.: Research Funding. Colombatti:Agios: Membership on an entity's Board of Directors or advisory committees; Global Blood Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees; Forma Therapeutics: Membership on an entity's Board of Directors or advisory committees; Vertex: Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees; Addmedica: Membership on an entity's Board of Directors or advisory committees; Italian Association of Pediatric Hematology Oncology (AIEOP): Membership on an entity's Board of Directors or advisory committees; Bluebird bio: Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees; European Haematology Association (EHA): Membership on an entity's Board of Directors or advisory committees. Waller:Novo Nordisk Healthcare A/G: Current Employment. Al-Behaisi:Novo Nordisk Healthcare A/G: Current Employment. Wufsus:Novo Nordisk Inc,: Current Employment, Current equity holder in publicly-traded company. Trimnell:Novo Nordisk: Consultancy; Pfizer: Consultancy, Honoraria; Agios Pharmaceuticals: Consultancy; Bluebird bio: Honoraria; Novartis: Consultancy; Vertex: Honoraria; Graphite Bio: Consultancy.